|
Navigation
|
8. Parkinson et lentiviraux. Avec quel(s) lentivirus? Avec quels résultats? Etudiants Adriana De Pesters / Jonathan Bernard --------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- La combinaison mots-clés/équations pertinentes en rapport avec le choix d'un outil est à revoir! 1/2 A. les mots-clés sont limités (voir suggestions en jaune). Il manque des suggestions de synonymes. 1/2 B. La grammaire des équations correspondant aux mots-clés n'est pas maîtrisée.
1/2 C. Outils: Plusieurs outils ont été explorés, mais les équations utilisées sont rarement pertinentes, ou ne fonctionnent pas en raison d'une synthaxe fausse. 2/2 D. Les références bibliographiques sont correctes, mentionnant avec quel outil elles ont été trouvées 1/2 E. Les éléments de réponse à la question contient des affirmations référencées, mais le copié-collé est tellement long, qu'il est difficile de voir les guillemets...pour le détail, on met la citation dans un texte, juste après les guillemets, mais pas à l'intérieur. Il faudrait réduire la taille des copier-coller, pour avoir quelque chose de plus pertinent. 1/2 F. La restitution écrite de la démarche de recherche d'information est difficile à suivre. Il manque de la précision pour que le lecteur puisse reproduire vos démarches, car la rédaction est un patchwork. Pour information, on peut utiliser des textes intégraux avec guillemets et source, ou des idées empruntées avec paraphrase et source (droit d'auteur). Mais la reproduction par copié collé d'image, de tableau, de dessin, de graphique, de statistiques, n'est pas possible, même en citant la source! Pour une reproduction, il faut demander aux auteurs et à l'éditeur la permission de reproduction (droit de diffusiont). Parfois, il faut payer pour obtenir ce droit. Autrement dit, le droit d'utilisation des "images" n'est pas le même que les idées sous forme écrite! 7 pts
Company: Oxford Biomedica
MORE INFORMATION ABOUT THE CLINICAL TRIAL
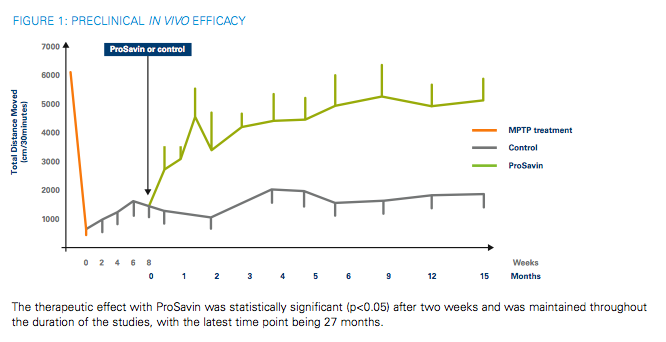
"The primary objectives of the trial are to assess the safety and efficacy of ProSavin. The analyses of patients will include the application of advanced non-invasive neuro-imaging techniques Patients in the trial will have been diagnosed with Parkinson's disease and will be failing on current treatment with L-DOPA but they will not have progressed to drug-induced dyskinesias. It is a two-stage study. The first stage is an open-label dose escalation to evaluate two dose levels of ProSavin in cohorts of three patients each. In the second stage of the trial, a further 12 patients will be recruited to confirm efficacy of the optimal dose. The efficacy of ProSavin will be assessed using the Unified Parkinson's Disease Rating Score (UPDRS). Patients will be monitored at regular intervals, with the primary endpoint being an efficacy assessment at six months after treatment. The secondary objective of the trial is to asses the extent to which patients' current therapy (L-DOPA) can be reduced following administration of ProSavin.2"
1. Source: Oxford Biomedica. < http://www.oxfordbiomedica.co.uk/ProSavin.asp >. Consulté le 08.11.08 2. Source: Clinical trials Gov. < http://clinicaltrials.gov/ct2/show/NCT00627588?term=gene+therapy+and+parkinson&rank=2 >.Consulté le 08.11.08
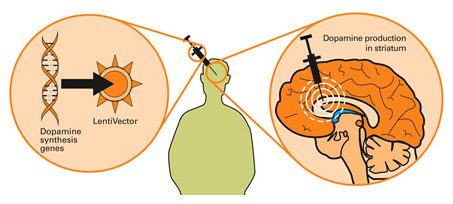
Note: There are several other research groups on other lentivirus to find a cure to neurodegenerative disease like PD. We can name as other vectors the HIV-1 virus or the SIN virus. Here we are not going to speak about these virus because there is no clinical trial with them for now, but you can still find some interesting references about them at the end of the page. Parkinson’s disease, gene therapy, genetic engineering, genetic therapy, drug delivery, delivery systems lentivir*, vector safety, toxicity, adverse effects company,industry clinical trials,clinical applications EIAV, Equine Infectious Anemia Virus Oxford biomedica
8.2 Equations testées dans quel outil de recherche documentaire Je ne comprends pas ce que sont <..>. La grammaire comprise en recherche documentaire est AND, OR, (), "", *, ?
Wikipedia
<Parkinson’s disease>, <lentiviral vector>, <gene therapy>, donc on pourrait écrire l'équation
"parkinson's disease" AND "lentiviral vector" AND "gene therapy"
Encyclopedia of cell technology <Lentivir* parkinson>
Pubmed <Parkinson AND lentivir*>
<Parkinson AND oxford AND biomedica> <lentivir* AND parkinson AND vector>
<Zufferey AND lentivir*>
Biosis
<Parkinson AND lentivir*> dans le TITLE (En restreignant tout de suite à une recherche dans le titre, on a seulement 3 résultats, pourquoi pas dans tous les champs? = 54 résultats)
<Parkinson AND (lentivir* OR gene therapy) AND (clinical trials)>
<Parkinson AND EIAV AND (clinical trials)> <Parkinson AND EIAV AND company AND (clinical trials)> <Parkinson AND EIAV AND (oxford biomedica) AND (clinical trials)> Google scholar
<Parkinson AND (clinical trial) AND eiav>, on pourrait écrire Parkinson AND "clinical trial" AND (EIAV OR lentivir*)
< Parkinson AND HIV-1>
8.3 Bibliographie suggérée Wong LF, Goodhead L, Prat C, Mitrophanous KA, Kingsman SM, Mazarakis ND. Romano G. Lawrence S Young *, Peter F Searle, David Onion, Vivien Mautner
Viral gene therapy strategies: from basic science to clinical application
The Journal of Pathology Early View Volume 208 Issue 2, Pages 299 - 318
Special Issue: Infection and Disease: Cause and Cure
Published Online: 17 Dec 2005
(google scholar)
Self-inactivating lentiviral vectors with enhanced transgene expression as potential gene transfer system in Parkinson's disease.
Déglon N, Tseng JL, Bensadoun JC, Zurn AD, Arsenijevic Y, Pereira de Almeida L, Zufferey R, Trono D, Aebischer P.
Hum Gene Ther. 2000 Jan 1;11(1):179-90.
PMID: 10646649 [PubMed - indexed for MEDLINE]
Viral vectors for in vivo gene transfer in Parkinson's disease: properties and clinical grade production
Mandel RJ, Burger C, Snyder RO.
Exp Neurol. 2008 Jan;209(1):58-71. Epub 2007 Aug 24. Review.
PMID: 17916354 [PubMed - indexed for MEDLINE]
About other vectors: HIV-1
The Journal of Immunology, 2005, 175: 112-123.
Copyright © 2005 by The American Association of Immunologists Michele A. Kutzler*, Tara M. Robinson
Coimmunization with an Optimized IL-15 Plasmid Results in Enhanced Function and Longevity of CD8 T Cells That Are Partially Independent of CD4 T Cell
The Journal of Immunology, 2005, 175: 112-123. Copyright © 2005 by The American Association of Immunologists
(google scholar) (pour référencer un périodique scientifique, sans éléments superflus, se référer au document guide des références bibliographiques)
About other vectors: self-inactivating (SIN) lentiviral vector
Self-inactivating lentiviral vectors with enhanced transgene expression as potential gene transfer system in Parkinson's disease.
Déglon N, Tseng JL, Bensadoun JC, Zurn AD, Arsenijevic Y, Pereira de Almeida L, Zufferey R, Trono D, Aebischer P.
Hum Gene Ther. 2000 Jan 1;11(1):179-90.
PMID: 10646649 [PubMed - indexed for MEDLINE]
|


 ---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------