- français
- English
Background Arsenic
Arsenic is the 33rd element which symbol is As in the periodic table and is a chemical element found in many mineral or in pure crystal forms. It is or was used in many industrial sectors as chemistry, electronics, metalworking, military, pharmaceutical, phytosanitary, mining, and others. Arsenic is also found in ground water, soil, air due to industrial or geological pollution and is found in many different forms and is often conjugated with sulfur and others metal. Almost all arsenic products are toxic but the inorganic ones are more dangerous. The AsO3, also called arsenic trioxide or arsenite, is one of the most common form in the environment because of it's important production in industry (around 50'000 tones per year). It's also a soluble form which thus can be ingested by drinking water which contain some. It explains why it's one of the most toxic form (until 500 times more toxic than pure arsenic).
The lethal dose for adult humans is between 70 to 200 mg. The biological activity of arsenic compounds interfere with the Krebs cycle were it inhibits the pyruvate conversion to acetyl-CoA. The world health organisation recommends a limit of 0.01 [mg/L] = 10 ppb in drinking water. This limit is based on the available detection limit. Intoxication occurs mostly by ingestion or inhalation of arsenic compounds. Acute intoxication is caused by ingestion of a large amount of arsenic compounds and causes vomiting, diarrhea, damage of the intestinal tract and dehydration. Chronic intoxication is cause by regular uptake of arsenic compounds and causes uncharacteristic symptoms that can last many years after end of exposure. Many forms of cancers like kidney, skin, lungs or liver cancers or anemia have been indexed. Others symptoms have been linked to arsenic intoxication as headache, drowsiness, cardiovascular problems, night blindness, hair loss, convulsions, vitamin A deficiency, reproductive problems, etc. 80 millions of people around the world daily uptake between 10 to 50 ppb in drinking water.
The first real test for arsenic detection, The Marsh test, was discovered by James Marsh in 1836, and was used to reveal arsenic presence in case of arsenic poisoning in forensic science. It was the first method which could trace specifically the presence of arsenic in the body. It uses sulfuric acid and zinc. Since a collection of different technics were developped.
For example, In 2009, At Jackson state university, the prof. Paresh Chandra Ray’s team developed a method using gold nanoparticles. Different organic molecules, which can grab arsenic ions, were attached on these nanoparticles. As up to three of these organic molecules can bind to one arsenic ion, the arsenic presence triggers formation of gold particles aggregate. As these particles turn from red color to blue color through these aggregation, the arsenic presence can be proved by colorimetry in a fast and simple way. It detects arsenic selectively and up to a concentration range of 1 ppb with naked eyes. But with a laser method called “dynamic light scattering”, the team was able to quantify the arsenic presence up to a concentration range of 3 ppt. The paper can be found here.
A similar approach was studied, at the beginning of 2013, by Indian scientists from Indian Association for the Cultivation of Science (IACS) which method uses gold clusters that emit light in presence of arsenic. This new technique is able to detect arsenic even in case of other metal ions presence.
The iGEM Edinburgh’s team won the 2006 competition’s best poster and real world application prices, and they were the third on the best device podium. They genetically modified an E. Coli strain in order to rearrange the bacteria’s natural arsenic detection and detoxification system with the natural lactose degradation system so that the bacteria, when sensing arsenic in their environment, begin to break the lactose from the culture solution down and thus acidify it. The arsenic quantification is then identified with a simple pH test. They want to develop a “simple, cheap and sensitive field assay for arsenic levels” from the technology they found. Unfortunately, the method takes about five hours before given statistically valid results and it’s not sure that these test are valid for real water samples with a lot of other molecules and particles.
Another approach for arsenic detection is developed by the Oxford University’s business program Isis Innovation. They are thus unclear in the involved method and only reveal that they use “modified glassy carbon electrodes electrochemical techniques. The advantage of this method seems to be that it detects low arsenic concentration even in copper (Cu(II)) presence which was a problem in the previous field test kits.
On the wagtech website, a specialist of water quality field test, There are at least two test for arsenic detection: The visual colour arsenic detection kit and the arsenator digital arsenic test. Both are low cost, portable, environmentally friendly, and simple to use field kits. The first one works visually, and compare the sample color after treatment to a list and detects until the range of 10 ppb. The second one gives valid results in 20 minutes, and detect in the range between 2 and 100 ppb.
A simple device which gives very rapid (15 to 120 second) and color-based result is sold on the detectors for heavy metal website.
A paper written in 2005 by Dan Kroll, a scientist chief from the Hach company, a society which products medical and test measurement equipment in colorado, propose a method which is now largely used throughout the world because of it’s simplicity and safety. The protocol, as the Marsh test, uses hydrogen zinc and sulfide oxidized in sulfate, to reduce arsenite to arsine gas, which is then transformed into arsenic-mercuric halogenid with help of mercuric bromide. This halogenid colors the test paper and shows the arsenic presence, and the contamination level is probably quantified in comparing the color, which varies between white-yellow-tan-brown, with a comparison list. This test kit has the advantage that it substitutes liquid hydrochloric acid with a powder form, which decreases random effects, linked with the halogenid’s liquid form. Another advantage is the new design of the test tube which retains the arsine gas, a toxic compound, in order to force it to react entirely into the halogenid form. It increases the test’s sensitivity and avoids the arsine gas release, which was a real problem some of the others test kits.
So, this very little list of arsenic detection tools, which regroup only a small part of the large detection device variety, shows that equally the industry and the academical world are interested in this research field and that there are many different ways of detections. The goals are now to ensure that no more people continue to drink arsenic-contaminated water, and to stop industrial arsenic pollution.
Many different bacteria have a constitutive arsenite and arsenate detection mechanism. It allows them to express a specific membrane protein complex which serves to pump the arsenite residues only when they are present. Arsenate can also be pumped out but is first reduced in arsenite with another enzyme. An arsenite sensing protein, called ArsR, normaly bond to a specific DNA region and inhibits the arsenite pump expression. When arsenite is present and bind to ArsR, it looses its DNA affinity so that the arsenite pump complex is then expressed. The biosensor use exactly this mechanism. the ArsR binding site was inserted into a plasmid followed by the fluorescent eGFP protein. This plasmid was then introduced in a strain of E. coli. The eGFP protein is thus expressed only in presence of arsenic and proportionally to its concentration. More information here.
In Switzerland, there are some geological contamination with arsenic, particularly in the massif of the Mont-Blanc, above Martigny. We also find some arsenic contamination in the Tessin where gold was formerly mined.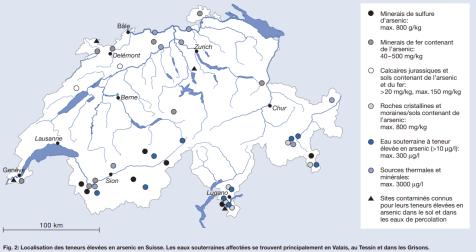 Image, from Hans-Rudolf Pfeifer and Jürg Zobrist, De l’arsenic dans l’eau potable, la Suisse également concernée? EAWAG news 53, p15-17. (Translation: Arsenic in drinking water: Switzerland also concerned?)
Image, from Hans-Rudolf Pfeifer and Jürg Zobrist, De l’arsenic dans l’eau potable, la Suisse également concernée? EAWAG news 53, p15-17. (Translation: Arsenic in drinking water: Switzerland also concerned?)
In this image, we see the places where water, hot water spring or ground soil are the most contaminated with arsenic. The purpose of the trip we will make is to visit one of these places and collect water.
