|
Navigation
|
6. Accidents causés par les vecteurs AAV (adeno-associated virus)Etudiants: Christian Pozzorini et Josephine Uldry -------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- Excellent, la méthodologie est pertinente, la rédaction agréable, et bien référencée! 2/2 A. les mots-clés sont pertinents 2/2 B. La grammaire des équations correspondant aux mots-clés est bien maîtrisée. Les équations sont pertinentes par rapport à l'outil sélectionné.
2/2 C. Outils: le type de documents trouvés dans divers outils de recherche est bien maîtrisé. Les outils sont multiples et complémentaires. Il y a une bonne pertinence entre les mots-clés, équations, choix des outils, et types de documents trouvés 2/2 D. Les références sont correctes 2/2 E. Les éléments de réponse à la question sont pertinents, et bien référencés. 2/2 F. La restitution écrite de la démarche de recherche d'information est claire, on comprend très bien les chemins méthodologiques empruntés! Pour information, on peut utiliser des textes intégraux avec guillemets et source, ou des idées empruntées avec paraphrase et source (droit d'auteur). Mais la reproduction par copié collé d'image, de tableau, de dessin, de graphique, de statistiques, n'est pas possible, même en citant la source! Pour une reproduction, il faut demander aux auteurs et à l'éditeur la permission de reproduction (droit de diffusiont). Parfois, il faut payer pour obtenir ce droit. Autrement dit, le droit d'utilisation des "images" n'est pas le même que les idées sous forme écrite! 12/12 pts
--------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 1. Qui sont les AAV et sont-ils dangereux?Mots-clés: AAV, gene therapy, adeno-associated virus, adverse effect, danger, death Pour une description simple et générale de la thérapie génique basée sur les AAV, les meilleures sources sont les livres, que nous trouvons via NEBIS ou les e-books, avec des équations assez simples. Si l'on s'intéresse aux dangers potentiels liés à l'utilisation des AAVs comme vecteurs, les sources techniques vulgarisées sont aussi très utiles. Enfin Google est aussi un bon outil pour ce chapitre, à condition d'avoir une équation bien construite. Quelques exemples de nos recherche:
a) E-book NEUROSCIENCEnetbase avec l'equation: AAV AND "gene therapy" "Adeno-associated virus (AAV) is a single-stranded DNA virus of the parvovirus family. It can be maintained as an episome or be integrated into the nuclear DNA. AAV is classified as a dependovirus because it requires a helper virus (adenovirus or herpesvirus) for active replication in infected cells. The required helper functions can be supplied in trans by packaging plasmids to make vector virus. AAV is particularly attractive for gene transfer because the wild-type virus has no known pathology in humans. The major limitation of AAV vectors is that they have a cloning capacity for foreign genetic sequences only of 4.5 kb." Source: Tyler Mark Pierson and John H. Wolfe. Gene Therapy For Inherited Diseases Of The Central Nervous System. In: Neurogenetics: Scientific And Clinical Advances. New York: David R. Lynch. 2005. On trouve deux livres potentiellement intéressants: un sur la thérapie virale pour le cancer notamment, et les techniques des vecteurs viraux, et l'autre sur l'animal cell technology comportant une partie sur les dangers ("Viral therapy of cancer" de K.J. Harrington et "Animal Cell technology" de K. Ikura. Comme nous n'avons pas les livres, nous ne savons pas les références exactes.)
c) Sciencedaily avec l'equation: AAV AND "adverse effect" "AAV vaccines against HIV may potentially cause harm and that, without additional pre-clinical studies, they should not be used in humans (...)" Source: The Wistar Institute. Virus Used To Create Experimental HIV Vaccines Directly Impairs Immune Response. ScienceDaily, 17 novembre 2007, Disponible sur http://www.sciencedaily.com/releases/2007/11/071115181814.htm (consulté le 9 novembre 2008).
d) Google avec l'equation: "gene therapy" AND "adeno associated" AND ("adverse effect" or "death")
"Recombinant adeno-associated virus (rAAV) vectors possess a number of properties that may make them suitable for clinical gene therapy, including being based upon a virus for which there is no known pathology and a natural propensity to persist in human cells.(...) Wild-type adeno-associated viruses (AAVs) are now known to be very diverse and ubiquitous in humans and nonhuman primates, which adds to the degree of confidence one may place in the natural history of AAV, namely that it has never been associated with any human tumors or other acute pathology, other than sporadic reports of having been isolated from spontaneously aborted fetuses. (...) On the basis of this understanding of AAV biology and a wide range of preclinical studies in mice, rabbits, dogs and nonhuman primates, a growing number of clinical trials have been undertaken with this class of vectors. Altogether, over 40 clinical trials have now been approved." Source: Mueller C., Flotte T.R. Clinical gene therapy using recombinant adeno-associated virus vector. Gene Therapy, 2008, 15, p.858-853., Disponible sur http://www.nature.com/gt/journal/v15/n11/full/gt200868a.html (Consulté le 07 novembre 2008).
Mots-Clés: gene therapy, adeno associated virus, adverse effect, death, Targeted Genetics, patient's death, Jolee Mohr Pour trouver des sources plus précisément sur les accidents liés aux AAVs, il faut affiner un peu notre recherche. Pour cela, nous avons d'abord trouvé avec google et l'équation du point 1.d le cas du décès d'une femme, Jolee Mohr, injectée avec des vecteurs AAV. Son nom nous permet d'affiner la recherche pour savoir les causes de l'accident. Pour cela, encore une fois, les sources vulgarisées sont très utiles. En plus, avec les mots clé que nous avons, nous pouvons tenter une recherche sur pubmed avec laquelle nous obtenons des données plus "scientifiques" sur le cas Mohr. Google scholar est aussi utile, puisqu'on y a trouvé qu'en plus du cas Mohr, une demi-douzaine d'autres patients sont morts des suite d'une injection virale, bien qu'il s'agisse de vecteurs adénoviraux et non d'AAVs. Il semble qu'il n'y a pas d'autres cas à déplorer incluant les AAVs. Quelques exemples de nos recherche:
a) The Scientist avec l'equation: AAV AND "Jolee Mohr"
"Mohr was part of Targeted Genetics' phase I/II clinical trial of tgAAC94, an adeno-associated virus (AAV) vector to treat inflammatory arthritis by inhibiting tumor necrosis factor at joints. Mohr died on July 24 2007. The subject, Jolee Mohr, 36, died from widespread histoplasmosis accompanied by a hematoma that ruptured her organs, according John Hart, a pathologist at the University of Chicago who presented the autopsy results. The cause of death of a patient in a July gene therapy trial was a massive fungal infection, according to an initial autopsy report presented at the NIH Recombinant DNA Advisory Committee meeting today (September 17). Preliminary evidence suggests that adeno-associated virus (AAV), the vector which delivered the therapy, was not to blame, experts said. (...)There have been 40 known cases of histoplasmosis in patients undergoing anti-tumor necrosis factor therapy." Source: Gawrylewski A. Panel reports on gene therapy death. The Scientist, 17 septembre 2007. Disponible sur http://www.the-scientist.com/news/display/53593/ (consulté le 07 novembre).
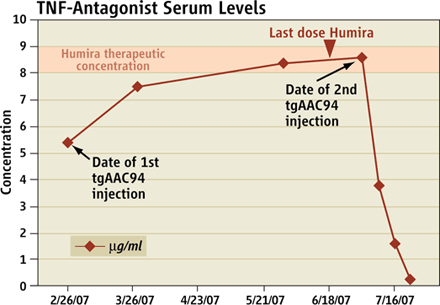
b) Pubmed avec l'equation: ("gene therapy" OR AAV) AND "patient's death" Ce graphique est une preuve scientifique que la concentration du serum injecté à Jolee Mohr n'a jamais dépassé la concentration thérapeutique.
Source: Kaiser J. Gene transfer an unlikely contributor to patient's death. Science, 07 décembre 2007, 318, 5856, p.1535. Disponible sur http://www.sciencemag.org/cgi/content/full/318/5856/1535 (consulté le 07 novembre 2008).
c) Google Scholar avec l'equation: ("gene therapy" OR AAV) AND "patient's death"
"Gene therapy is once again in the spotlight—brought there by news reports of "adverse events," including half a dozen deaths, involving participants in several separate clinical trials. Although none of these deaths has yet been attributed directly to the gene transfer procedures being evaluated, the mere possibility of such a connection is stirring intense interest, and highlights some of the complications that arise from dual oversight of the field. (...)The immediate focus is on the safety of vectors that are based on genetically modified versions of human adenovirus, several of which are being used to deliver genes into patients involved in a variety of clinical trials, including those in which the recent deaths occurred." Source: Fox J. Gene therapy safety issues come to fore. Nature biotechnology, 1999, 17, p.1153. Disponible http://www.nature.com/nbt/journal/v17/n12/full/nbt1299_1153.html (Consulté le 07 novembre 2008).
Mots-Clés: gene therapy, adeno associated virus, patient's death, Jolee Mohr, tumour-necrosis factor, TNF, tgAAC94 Pour savoir en détail la biologie de l'accident (gène et vecteur responsables), nous avons cherché sur pubmed. Notre recherche:
a) Pubmed avec l'equation: "gene therapy" AND "patient's death" "Although DNA sequences from the vector were found in her liver and spleen, "the detection of the sample is very low and below the limit of quantification in these assays", said Jeffrey Bartlett, a member of the National Institutes of Health (NIH) Recombinant DNA Advisory Committee (RAC). "It really indicates the absence of ongoing replication of the vector in these tissues. (...) Mohr died on 24 July, 22 days after her right knee was injected with an adeno-associated virus (AAV) vector made by Targeted Genetics, a Seattle company. This vector was engineered to contain a gene for an anti-inflammatory protein, TNFR:Fc, which inhibits tumour-necrosis factor- (TNF-). TNF- causes inflammation in rheumatoid arthritis. (...) TNFR:Fc suppresses the immune system, and so it is possible that the gene therapy caused the infection. However, the design of the trial, in which some patients were also taking similarly acting immunosuppressant drugs, means that it will be difficult to ascertain what caused the infection to take hold. Mohr was also taking a TNF- Source: Wadman M. Gene therapy might not have caused patient's death. Nature, 20 septembre 2007, 449, p.270. Disponible sur http://www.nature.com/nature/journal/v449/n7160/full/449270b.html (consulté le 07 novembre 2008).
Mots clés: Jolee mohr, seattle, judgement, responsibility, trial... Pour ce chapitre, nous pouvons affiner notre recherche avec quelques mots clés supplémentaires (trial, judgement, responsibility). Le mot clé "trial" associé à "Jolee Mohr" donne les meilleurs résultats. Comme nous nous intéressons à un sujet peu spécifique et moins scientifique que pour le point 3, les sources vulgarisées sont à nouveau utiles. Quelques exemples: a) lexisnexis avec l'equation: "jolee mohr" AND (trial OR responsiblility OR judgement) "The July death of agene therapy clinical trial participant primarily was the result of disseminated histoplasmosis with subsequent bleeding complications and multiorgan failure, federal advisers said recently. However, the National Institutes of Health Recombinant DNA Advisory Committee (RAC) could not completely rule out Targeted Cenetics'tgAAC94 as a possible contributor in the death of the 36-year-old Illinois woman. (...)" Source: BioWorld Week. NIH panel won't rule out gene therapy in death. 10 décembre 2007, 15, 50, p. 3. Disponible sur http://www.lexisnexis.de/e-solutions/KSH/en/index.html (Consulté le 09.11.2008). b) Google avec l'equation: "jolee mohr" AND seattle AND (trial OR responsiblility OR judgement) "The FDA's decision clears some concern about the future of gene therapy, a field considered promising but risky. It also helps restore confidence in Target Genetics' clinical pipeline, which relies on gene-therapy products."This is very good for the company, for the product and for gene therapy," said Chief Executive H. Stewart Parker. But Mohr's death also raised issues about whether patients with nonlethal diseases were being properly informed of the risks of medical experimentation before consenting to participate in clinical trials. Targeted Genetics said that it was revising the "informed consent" document patients review before enrolling to include information about Mohr's death. The company is also amending the study's procedure to keep doctors from administering the drug to patients suffering from fever at the time scheduled for the injection. Mohr had a mild fever when she was injected with the treatment." Source: Gonzalez A. Targeted Genetics restarts trial after woman's death. Seattletimes, 26 novembre 2007. Disponible sur http://seattletimes.nwsource.com/html/businesstechnology/2004035019_targetedgenetics26.html (Consulté le 09 novembre 2008). c) Google scholar l'equation: jolee mohr AND (trial OR judgement OR responsibility)
"(...) Federoff and others say other question remain unanswered, such as whether Mohr realized that patients weren’t expected to benefit from this study". Source: Kaiser J. Question remain on cause of death in arthritis trial. Science, 21 septembre 2007, 317, p.1665. Disponible sur http://www.sciencemag.org/cgi/reprint/317/5845/1665a.pdf (Consulté le 09 novembre 2008).
Mots-clés: "clinical trials", "Targeted Genetics", "gtAAC94", "food and drug administration", "hold on", "suspended" Pour répondre à cette dernère question, nous avons comencé par checher sur Google. Cette stratégie a fourni des résultats probants. a) Google avec l'equation: "food and drug administration" AND "targeted genetics" AND suspended
Source: News In Brief. Gene-Therapy tial on hold. Nature Reviews Drug Discovery, septembre 2007, 6, p. 690-691. Disponible sur: http://www.nature.com/nrd/journal/v6/n9/full/nrd2417.html (consulté le 09 novembre 2008).
b) Google avec l'equation: "food and drug administration" AND "targeted genetics AND "hold on" "Back in July, a Phase I/II study was halted after patient Jolee Mohr died [...] The US Food and Drug Administration has allowed Targeted Genetics to restard testing its arthritis gene therapy after deciding the drug wasn't at fault when one person died in the original clinical trial [...] The 35 patients who have not yet received their second dose of tgAAC94 will be shown an updated consent form, which includes information about Mohr's death and patients will not be allowed to receive the treatment if they are suffering from a fever at the time of injection, as Mohr was." Source: Nagel M. Targeted Genetics restarts trial after death. Breaking news on drug discovery, 26 novembre 2007. Disponible sur http://www.drugresearcher.com/Emerging-targets/Targeted-Genetics-restarts-trial-after-death (consulté le 09 novembre 2008).
c) Google avec l'equation: "targeted genetics AND "completed" AND "clinical trials" "SEATTLE, WA and SAN FRANCISCO, CA, Oct 27, 2008 (MARKET WIRE via COMTEX News Network) -- Targeted Genetics Corporation (NASDAQ: TGEN) announced positive results of its now completed Phase 1/2 clinical study with the last subject having finished the protocol-required follow-up. The data demonstrated that tgAAC94, an investigational agent designed to inhibit activity of tumor necrosis factor-alpha (TNF-alpha), a key mediator of inflammation, is well tolerated and may improve disease symptoms in inflammatory arthritis patients." Source: Byars S.D. Targeted Genetics announces presentation of data from completed phase 1/2 TGAAC94 trial at American College of rheumatology. 27 octobre 2008. Disponible sur http://ir.targen.com/phoenix.zhtml?c=84981&p=irol-newsArticle&ID=1217936&highlight= (Consulté le 09 novembre 2008).
Bibliographie (pour les moteurs de recherche, se référer aux chapitres):
1. BioWorld Week. NIH panel won't rule out gene therapy in death. 10 décembre 2007, 15, 50, p. 3. Disponible sur http://www.lexisnexis.de/e-solutions/KSH/en/index.html (Consulté le 09.11.2008) 2. Byars S.D. Targeted Genetics announces presentation of data from completed phase 1/2 TGAAC94 trial at American College of rheumatology. 27 octobre 2008. Disponible sur http://ir.targen.com/phoenix.zhtml?c=84981&p=irol-newsArticle&ID=1217936&highlight= (Consulté le 09 novembre 2008). 3. Fox J. Gene therapy safety issues come to fore. Nature biotechnology, 1999, 17, p.1153. Disponible http://www.nature.com/nbt/journal/v17/n12/full/nbt1299_1153.html (Consulté le 07 novembre 2008). 4. Gawrylewski A. Panel reports on gene therapy death. The Scientist, 17 septembre 2007. Disponible sur http://www.the-scientist.com/news/display/53593/ (consulté le 07 novembre). 5. Gonzalez A. Targeted Genetics restarts trial after woman's death. Seattletimes, 26 novembre 2007. Disponible sur http://seattletimes.nwsource.com/html/businesstechnology/2004035019_targetedgenetics26.html (Consulté le 09 novembre 2008). 6. Kaiser J. Gene transfer an unlikely contributor to patient's death. Science, 07 décembre 2007, 318, 5856, p.1535. Disponible sur http://www.sciencemag.org/cgi/content/full/318/5856/1535 (consulté le 07 novembre 2008). 7. Kaiser J. Question remain on cause of death in arthritis trial. Science, 21 septembre 2007, 317, p.1665. Disponible sur http://www.sciencemag.org/cgi/reprint/317/5845/1665a.pdf (Consulté le 09 novembre 2008). 8. Mueller C., Flotte T.R. Clinical gene therapy using recombinant adeno-associated virus vector. Gene Therapy, 2008, 15, p.858-853., Disponible sur http://www.nature.com/gt/journal/v15/n11/full/gt200868a.html (Consulté le 07 novembre 2008). 9. Nagel M. Targeted Genetics restarts trial after death. Breaking news on drug discovery, 26 novembre 2007. Disponible sur http://www.drugresearcher.com/Emerging-targets/Targeted-Genetics-restarts-trial-after-death (consulté le 09 novembre 2008). 10. News In Brief. Gene-Therapy tial on hold. Nature Reviews Drug Discovery, septembre 2007, 6, p. 690-691. Disponible sur:http://www.nature.com/nrd/journal/v6/n9/full/nrd2417.html (consulté le 09 novembre 2008). 11. The Wistar Institute. Virus Used To Create Experimental HIV Vaccines Directly Impairs Immune Response. ScienceDaily, 17 novembre 2007, Disponible sur http://www.sciencedaily.com/releases/2007/11/071115181814.htm (consulté le 9 novembre 2008) 12. Tyler Mark Pierson and John H. Wolfe. Gene Therapy For Inherited Diseases Of The Central Nervous System. In: Neurogenetics: Scientific And Clinical Advances. New York: David R. Lynch. 2005. 13. Wadman M. Gene therapy might not have caused patient's death. Nature, 20 septembre 2007, 449, p.270. Disponible sur http://www.nature.com/nature/journal/v449/n7160/full/449270b.html (consulté le 07 novembre 2008).
|

 -inhibitor drug for the arthritis, which suppresses the immune system. (...) Officials at the NIH and the US Food and Drug Administration reported at the meeting that they had reviewed all the AAV trials to date and found no pattern of adverse events associated with AAV, or any increased incidence of adverse events compared with trials using other gene-therapy vectors. The trial Mohr took part in was unusual in that it involved repeated injections. It was halted after her death."
-inhibitor drug for the arthritis, which suppresses the immune system. (...) Officials at the NIH and the US Food and Drug Administration reported at the meeting that they had reviewed all the AAV trials to date and found no pattern of adverse events associated with AAV, or any increased incidence of adverse events compared with trials using other gene-therapy vectors. The trial Mohr took part in was unusual in that it involved repeated injections. It was halted after her death."