- français
- English
Estimating the glycerin's water content through rheometry
In order to verify if our viscosity approximation was correct, we tested our water and glycerine mixture with one of the lab's rheometers.
We will not go into the details of how a rheometer works, but is quite smart and we encourage you to have a look at it. In practise, we simply put a drop of our mixture in the machine, and an increasing strain rate is applied. Using basic continuum mechanics, it is possible to extract the fluid's viscosity by measuring the force required to maintain the strain rate. The results are presented in the Figure below.
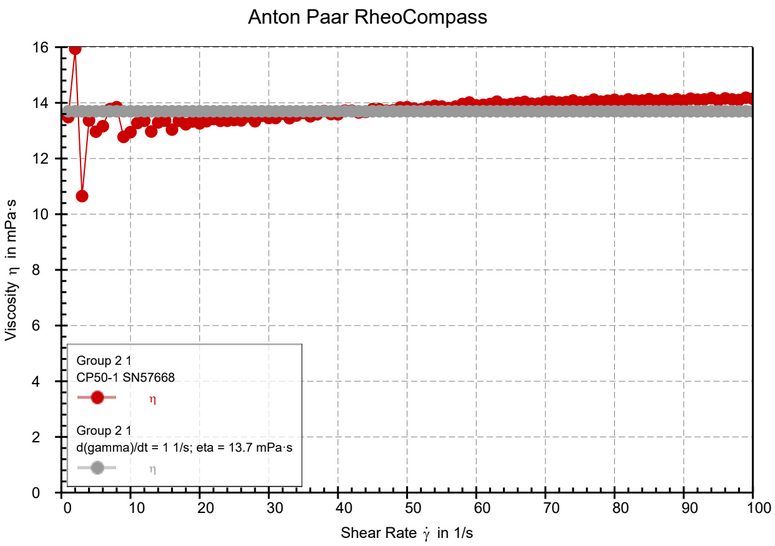
We were aiming for a viscosity of 21,4 mPa s, but the rheometer indicates we are in fact standing at 13.7 mPa s on average. This came to us as a surprise, since this35% difference cannot be explained solely by imprecisions in making the sample or temperature differences. This is corroborated by the fact that preliminary particle tracking results give us a viscosity of around 15 mPa s.
The only plausible explanation is then that the glycerine was not pure, meaning that it contains a certain amount of water. Using the online tool presented in class, one can estimate that the glycerine we used contains around 10% water. This is a rough estimate whose discussion is left to the reader, and which will help make sense of some discrepancies between the theoretical estimate and the experimental results.
Update by the author (07.01): 10% water in the glycerine is a lot, so we want to mention the fact that when pipetting glycerine, it is easy to capture air bubbles, which could explain some of the viscosity drop (not the whole 35.5%, still).
