|
Navigation
|
[The page serves a knowledge base for the known mechanism in the chemotaxis signal transduction in E. coli, in particular for the clustering of the chemoreceptors]
1. Introduction The chemotaxis signal transduction pathway in E.coli is an exceptionally well characterized pathway. The large amount of quantitative data triggered several attempts to mathematically model the systems. Although many aspects of the system, such as the adaptation mechanism were captured, the function of the observed clustering of receptors and its potential relation to the remarkable sensitivity of the chemotaxis system remain elusive. The involved chemoreceptors are transmembrane receptors and are also classified as methyl-accepting chemotaxis proteins (MCP). The residuals in the cytoplasmic part of the receptors that can be methylated are believed to be responsible for the adaptation of the signaling systems and modulate the ligand affinity of the receptors. They are also supposed to be related to structural properties of receptor, i.e., whether the long cytoplasmic part is more stiff or more flexible/dynamic. The two predominant receptor types are those for aspartate (Tar) and serine (Tsr), with several thousands of molecules per cell. Less abundant are receptors for dipetides (Tap), ribose and galatose (Trg) and redox potential (Aer) with a few hundreds per cell. The basic story of the pathway is as follows. A repellent or and attractant ligand binds to the periplasmic part of the receptor. To relay this signal further the histidine kinase CheA and the adaptor protein CheW is required. CheW binds to cytoplasmic tip of the receptor and binds with CheA. That is the basic timeric configuration. The autophosphorylation activity of CheA is inhibited by attractant binding and enhanced by repellant binding. The phosphoryl group from activiated CheA is quickly transferred to the reponse regulator CheY. The ratio between phosphorylated CheY (CheY-P) to unphosed CheY determines the rotation direction of the flagella motors. CheY-P at the flagella motors cause a change in rotation direction from counterclockwise to clockwise, which promotes the tumbling movement. Thus binding of repellants causes tumbling and thus a potential reorientiation. CheY-P gets dephosed quickly by the phophatase CheZ garantueing a short response time. If there is no feedback then in the presence of a repellant ligand the system keeps tumbling. The necessary feedback is implemented over the methylation of the glutamyl residuals (4 residuals per receptor monomers, receptors come as dimers always). This receptor modification is implemented via the methyltransferase CheR and the methylesterase CheB adding and removing methyl groups from the receptor, respectively. An increase in methylation level of the receptor causes an increase of CheA activation and decrease in receptor sensitivity with respect to attractants. The methylation process is modulated by the activity of CheA. That is CheB can be phosphorylated by active CheA and therewith increases its acitivity as a methylesterase. Thus an increase of attractants first causes a decrease in active CheA, that in turn causes a slower demethylation resulting in a higher net methylation level of the receptor. With the above said, this higher methylation causes lower receptor sensitivity with respect to attractants AND an increased CheA activity. So possibly, in the presence of that higher attracant level the number of active CheA returns to its level before the increase of attractants. This has been extensively studied and is not the main focus of that investigation. See below a schematic of the discussed interaction.
Here we are more interested in an explanation for the observed clustering of the receptors. See below the cool experimental evidence of such an cluster or patch formation.
2. Clustering There is good evidence that the basic functional unit of the receptors is a trimer of receptor dimer and that the tips of the cytoplasmic end of those three receptors can bind, independent of the presence of CheW or CheA. It was shown with Nanodisc embedded chemoreceptors that a single receptor in a Nanodisc is capable of binding the ligand, transmitting the signal to the cytoplasmic part, to have adaptation via the methylation sites, but it was not able to activate CheA. Suprisingly in preparation of Nanodiscs involving differently many receptors, the acitivity of CheA versus the number of those embedded receptors peaked at 3, the number that is also supported from other experimental findings. See below a picture of one or three receptor homodimers embedded in a Nanodisc.
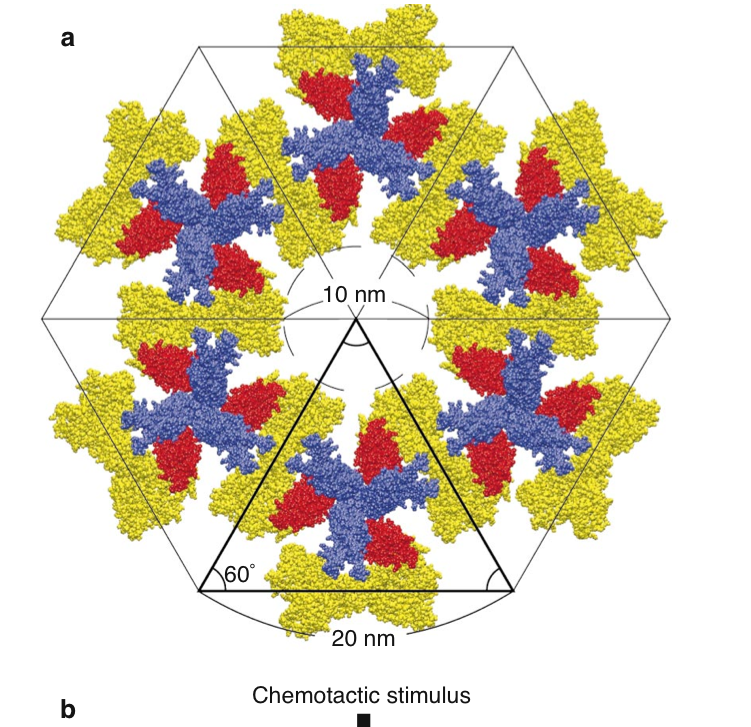
In 2000 Shimizu et al. proposed an intriguing model for receptor clustering. First, some facts about the involved agents. The adaptor CheW is a small monomeric protein and is supposed to bind the receptor tip as well as CheA. CheA forms dimers (the monomers come in two types, i.e., full length CheAL and shorter CheAS; more later). A single CheA monomer has 5 domains, i.e., P1 phos domain, P2 the CheY and CheB binding site, P3 the dimerization domain, P4 catalytic domain, P5 regulatory domain, that allows to couple CheA to the receptor and to CheW. Analyzing the 3D protein structure Shimizu et al found that CheW fits into the groove between two receptor homodimers. The proposal is now that 3 CheW bind the three grooves in a trimer of dimer receptorbundle and that at each CheW one part of the CheA dimer is bound in the P5 site. Thus we have the stoichiometry in terms of monomers of ( 6 x receptor ) : ( 3 CheW ) : (3 CheA ). The second P5 site of the CheA dimer is bound with a neighboring receptor trimer. Based in this triangle structure we can build hexgonal shapes as indicated below and thus a lattice of arbitrary size.
Left: View parallel to the membrane; trimer of receptors (blue), CheW (red) and CheA dimers (one monomer yellow, one gray); CheW binds the receptor at the cytoplasmic end. Right: Assembly of the basic trimeric unit into a hexagon; Dimeric CheA links different receptor trimers with each other.
3. Model 0 Based on the above observations one can construct a simple rule-based model for the clustering alone. We assume that the proposed receptor unit of a trimer of receptor homodimers has formed and we spend three binding sites for the adaptor CheW, i.e., trimeric agent Tar3(r1,r2,r3). We assume for the monomeric adaptor protein CheW two binding sites, i.e., agent CheW(s1,s2). Following the experimental evidence we spend two binding sites for the dimer CheA, i.e., agent CheA(x1,x2). The kappa rule set giving rise to an approximate hexagonal lattice is then # Binding of the adaptor CheW to the receptor trimer Tar3(r1),CheW(s1) <-> Tar3(r1!1),CheW(s1!1) @ k1_on, k1_off # recruitment of CheA by CheW (possible already at the receptor) CheW(s2),CheA(x1) <-> CheW(s2!1),CheA(x1!1) @ k2_on, k2_off # initial conditions such we have a total pairing
It give a approximate regular structure because there is the change that the second site of the CheA that should link to another receptor trimer over a CheW, actually links back to the original receptor trimer. The graphical stationary state of the ensemble is given below. The structure is not regular and hexagonal because a standard graph layout algorithm was used. Nevertheless it seems that the mentioned self-loops are not occuring very frequently. They could be avoided by considering steric constraints.
Some references so far: @article{shimizuts_2000_1, @article{borrokmj_2008_1,
|

 (Hazelbauer 2008)
(Hazelbauer 2008)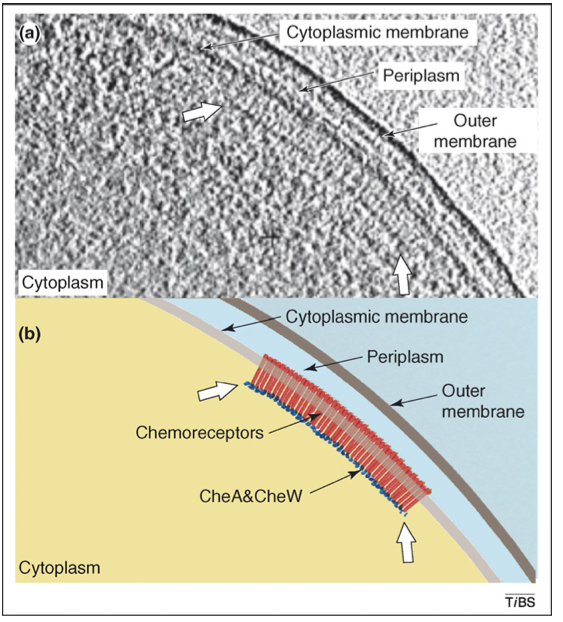 Hazelbauer 2008, Subramanian 2007)
Hazelbauer 2008, Subramanian 2007)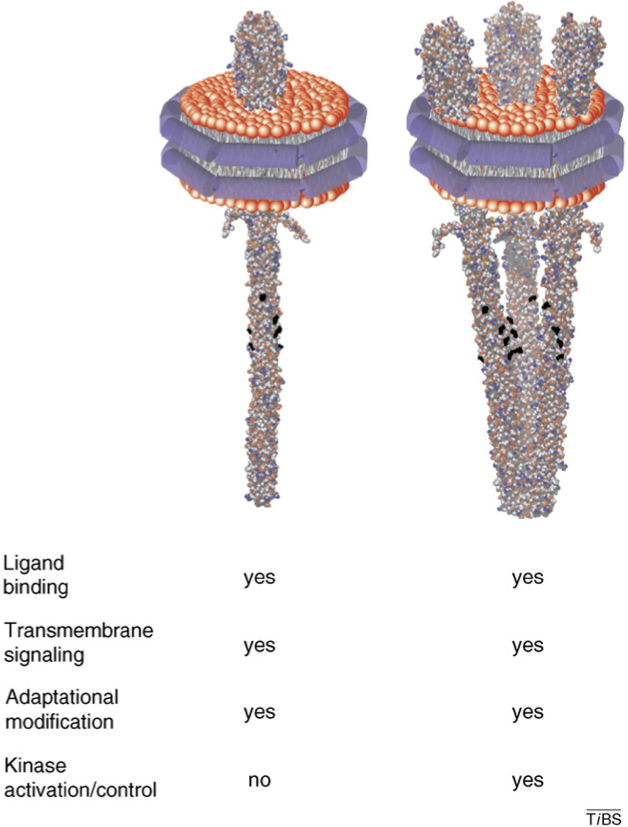 (Hazelbauer 2008)
(Hazelbauer 2008)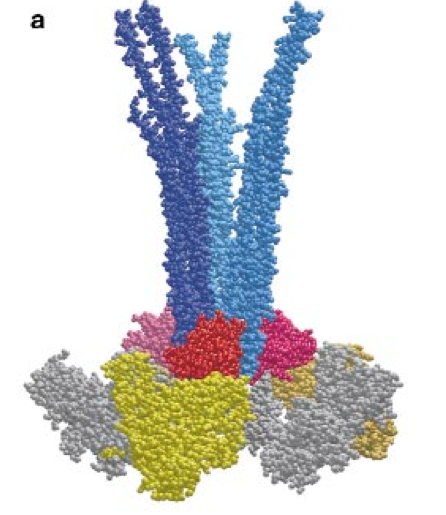
 (Bray et al. 2000)
(Bray et al. 2000)