- français
- English
Nmr
WELCOME TO THE ISIC's NMR SERVICE WIKI
We hope you will find here answers to your questions regarding the NMR service of the Institut des Sciences et Ingénieurie Chimiques de l'EPFL. If you need some complement, do not hesitate to contact us at pascal.mieville"at"epfl.ch.
Table of Content (links):
1. Available NMR spectrometers and ideal use
2. Useful links
3. Data access and account management
4. Routine TOPSPIN commands for manual machine
5. Unlock Screen saver
6. MestreNOVA
7. Automatic structure verification
8. NMR references tables
9. Useful NMR tricks
10. Kinetic experiments
1. Available NMR spectrometers and ideal use
Table of all available probes and machines at epfl © Martial Rey
Table of Signal to Noise ratio © Martial Rey
| LOCAL | SPECTROMETER | TYPICAL APPLICATION | CRYOPROBE |
|---|---|---|---|
| BCH 1508 |
Avance 500 MHz |
Low concentration samples |
yes (Helium) |
| BCH 1510 |
AvanceII 800 MHz |
Liquid: High resolution, low concentration samples (bio) Solid: 1H, 13C,14N, NMR development |
yes (He) factor 4 in sensitivity |
| BCH 1512L |
DRX-400 MHz |
1H,13C, BB, good sensitivity on carbon and other low gamma nuclei such as 31P
NMR development or routine |
no |
| BCH 1512R |
Avance 400 MHz
|
1H, {BB}, very good sensitivity on proton, simple gradient, medium sensitivity on broad band. |
no |
| BCH 1516 |
AVANCE III HD 600 MHz |
Low concentration samples |
yes (He) factor 4 in sensitivity |
| BCH 2407L |
Avance I 400MHz |
Good routine machine for 1H, 13C, 19F and 31P | no |
| BCH 2407R |
DRX 400 MHz |
Special machine for high pressure measurments | no |
| BCH 3204 |
DRX 400 MHz |
!! GOOD for 1H (zg and COSY) and all reverse experimet |
no |
| BCH 3404L |
AvanceIII 400MHz |
Good routine machine for 1H, 13C, 19F and 31P. |
no |
| BCH 3404R |
Avance 200MHz |
Low resolution 13C, 19F, 31P. Good for kinetic measurements |
no |
|
BCH |
AvanceIII 400MHz (nmr4313L.epfl.ch) |
AUTOMAT, high sensitivity in 1H, 19F, 13C and 31P, good for characterization.
Priority for less than 15 minutes. |
yes (prodigy, N2) factor 2 in sensitivity |
| BCH 4313R |
AvanceIII 400MHz Smartprobe 5mm ICONNMR ATMA, TOPSHIM, AUTOSAMPLER (nmr4313L.epfl.ch) |
AUTOMAT,very good sensitivity in 1H and 19F. Good sensitivity in 13C and 31P. |
no |
| BCH 5313 | AvanceIII 400MHz BBFO-Plusz 5 mm TOPSPIN 3 ATMA, TOPSHIM, (nmr5313.epfl.ch) |
Good routine machine for 1H, 13C, 19F and 31P. |
no |
| CH F0492L | AvanceIII 400MHz BBFO-Plusz 5 mm TOPSPIN 3 ATMA, TOPSHIM, (nmrCHF0492L.epfl.ch) |
Good routine machine for 1H, 13C, 19F and 31P |
no |
| CH F0492R |
Avance 400 MHz |
Good routine machine for 1H, 13C, 19F and 31P | no |
2. Useful links and documents
Please, if you use information coming from the following sites, be careful to correctly cite them in your references.
- The essential lists of solvent traces and chemical shift references :
J. Org. Chem. 1997, 62, 7512-7515 (contains also chemical shift HDO vs T°)
Organometallics 2010, 29, 2176–2179
Magn. Reson. Chem. 2006; 44: 606–616 (contains 2H values and TMS)
- Our estimated colleague Dr. Luc Patiny's tools for NMR prediction and simulation :
http://www.nmrdb.org/predictor
http://www.nmrdb.org/simulator
- An excellent reference about NMR nuclear properties of many elements from Roy Hoffman at Hebrew
University of Jerusalem : http://chem.ch.huji.ac.il/nmr/techniques/1d/multi.html
- A famous and very useful spectral database from Japan :
http://sdbs.riodb.aist.go.jp/sdbs/cgi-bin/cre_index.cgi
- Bruker software (and updates) - good prices for student licenses on TOPSPIN and CMC-assist
http://www.bruker.com/service/support-upgrades/software-downloads/nmr.html.
- A useful Solid State NMR link :
http://www.solidstatenmr.org.uk/lectures.html
- A good reference for structure elucidation : Richards, S. A., Hollerton, J. C. Essential Practical NMR for Organic Chemistry; John Wiley and Sons: United Kingdom, 2011; 157-159. ISBN: 978-0-470-71092-0
- A NMR blog maintained by Dr. Glenn Facey from Ottawa University that contains a lot of very useful and smart tricks and good explanations about common NMR experiments :http://www.u-of-o-nmr-facility.blogspot.ch/
3. Data access and account management
3.1 For EPFL registered users (possessing a GASPAR account)
3.1.1. To access data directly from your own computer:
On any machine type (windows, mac & Linux) it is possible to use a SFTP software like FileZilla to connect.
 Here is the link to download FileZilla (it is free and easy to use) : https://filezilla-project.org
Here is the link to download FileZilla (it is free and easy to use) : https://filezilla-project.org
Once installed on your machine, apply first a small correction into the preferences :
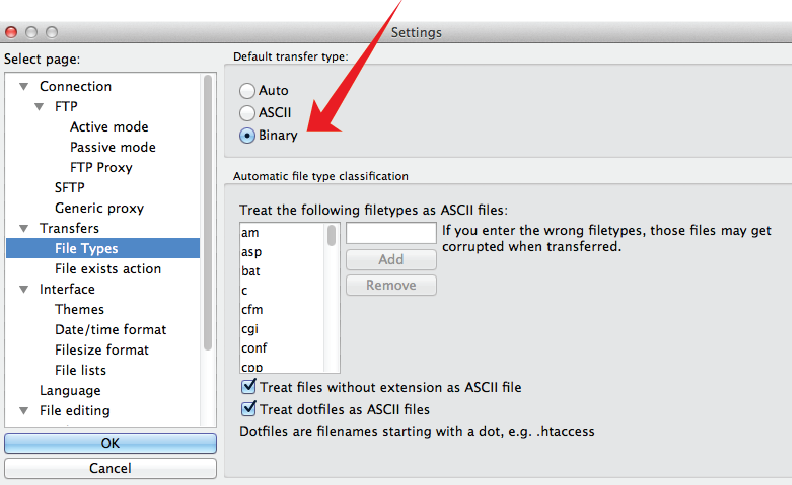
- In the Menu Bar, go to FileZilla, then choose Preferences.
- In the Setting window, choose the Tranfers -> FileTypes options.
- In the upper window (Default Transfer Type) choose the "Binary" option. This will avoid any modification of the files during the file transfer.
Then connect with the following information:
Host : nmr.epfl.ch
Username : your GASPAR username
Password : your GASPAR password
Port : 22 (new for more security)
Finally press : Quickconnect
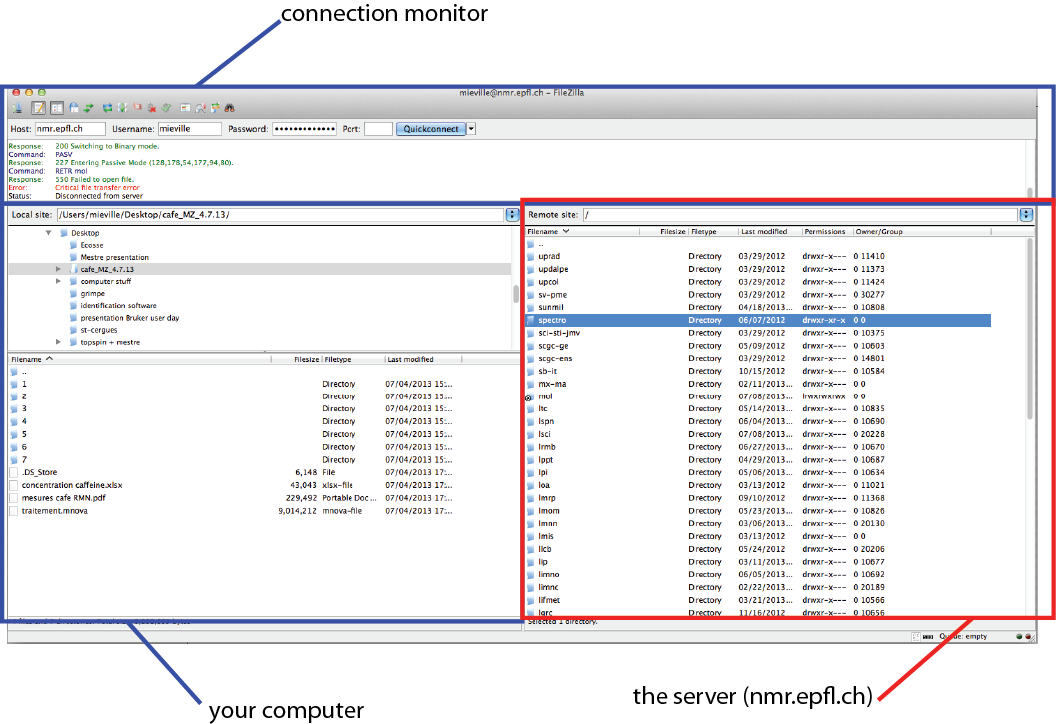
In the right window (red one, server) choose the spectro link, then choose the correct spectrometer. You should have all your data available. You can therefore drag them in your computer (left window, blue).
It is also possible to connect directly to the server using the drive mapping process, see below.
including one called Map network drive.
folder you want to connect to. In our case \\nmr.epfl.ch\isic
Therefore you have to validate the license (click "Agree"), then in the left vertical bar, click the left mouse button, then select "Add new directory" and type "level_#" (replace # by your specific level number).
are similar to the former procedure. If your folder does not exist already, it will be generated automatically at this step.
For confidentiality reasons, we do not store nor backup any external user data. We therefore ask GUESTS users to directly retrieve their data after acquisition and to erase them from the spectrometer (see 2.2.2).
Dedicated folders are specifically not backed-up.
NMR service can not be considered as responsible in case of loss or diffusion of data that have not been correctly removed from the spectrometer by external/guests users.
The two dedicated machines for external users are nmr1512l and nmr1512r.
3.2.1 Data generation
In TOPSPIN or XWINNMR, type "EDC" or "NEW", in the popup window fill the following information :
NAME = EXPERIMENT_NAME
EXPNO = 1
PROCNO = 1
DIR type : /opt/data/guests (this folder is in mode 770, root : 500000)
This will generate data into the following folder : /opt/data/guests/data/USER/nmr/...
3.2.2 Data recovery using FileZilla
A dedicated MAC machine is available in the BCH 1512 room. This machine is equipped with FileZilla
Connect yourself to the server using FileZilla, connexion information are host = nmr.epfl.ch , user = USER (e.g. G13000, G has to be capital) and you password (port 21 = FTP mode).
The right window in FileZilla is the remote machine (server) and the left window is the local machine. Your USB key should be visible in the left window under "Volume" folder.
Data are available on the right window under path : /spectro/MACHINE/guests/data/USER/nmr
Copy all data simply by drag and drop from server to your key, then suppress all data on the server. This will also suppress them on the spectrometer.
Finally, in FileZilla, erase your connexion data using the small arrow on right of "connexion rapide" and clicking on "effacer historique".
4. Routine TOPSPIN commands for manual machine
download routine TOPSPIN commands for manual machine
5. Unlock Screen Saver
Under the new LDAP configuration (GASPAR login), the screen saver has to be set individually. In order to avoid to block the machine in case of absence, please configure the screen saver to not request password to unlock.This has to be done in the linux configuration as described hereafter:
5.1 On CentOS 
a. Go to the "CENTOS" menu, then select "Control Center" -> "Appearance & Themes" -> "Screen Saver"
b. In the "Screen Saver" window, uncheck the checkbox "Request Password to stop"
5.2 On RedHat 
a. Go to the " REDHAT Application" menu (upper left corner), then select "Preferences" -> "Screen Saver"
b. In the "Screen Saver" window, change "mode" to "Disable screen saver".
This licenses are only destinated to ISIC (Chemistry) users. All other will be banned when detected on the license server.
All ISIC collaborators have access to a license site for MestreNOVA NMR, Mestre NOVA predict and two add-ons : qNMR (for quantitation) and Reaction Monitor.
The link to download the main software is http://mestrelab.com/software/mnova-suite/download/
6.1 Install Mnova
Go to the "Mestre License Download" tab on http://isic.epfl.ch/NMR
qNMR add-on can be downloaded here: qNMR add-on
Reaction Monitor add-on can be downloaded here: Reac_Monitor_add-on
To install the add-ons, download them on your desktop, unzip them and drag the .xml file into the MestreNova open window.
7. Automatic structure verification
ISIC has bought two softwares/licenses dedicated to help users to evaluate the consistency of their spectra. A first soft is included in ICONNMR on the automatic NMR machines (see 7.1) and does not requires installation on the user machine. A second soft is MestreNova Verify and is an add-on to Mestre (see 7.2).
These tools are not intended to replace the chemist but only to support him. It is always the chemist at the end that has to decide if the spectra is good or not.
A good reference for structure elucidation : Richards, S. A., Hollerton, J. C. Essential Practical NMR for Organic Chemistry; John Wiley and Sons: United Kingdom, 2011; 157-159. ISBN: 978-0-470-71092-0
7.1 CMC-assit Fast Lane on Bruker automatic machines (nmr3404l, nmr4313l & r and nmrf492l)
go to the following page : Bruker Fast-Lane, to have a complete description to work with CMC-assist
Fast Lane
here is a reference manual for CMC-assist (pdf) - not really required to read it.
7.2 MestreNova Verify
On specific request, ISIC users only can obtain a license for Mnova Verify. To do this request please click on the following link : https://isic-nmr.epfl.ch/licenses-request/verify
More information about this software : http://mestrelab.com/software/mnova-verify/
A MestRe site treating of Automatic Structure Verification/Elucidation
http://mestrelab.com/blog/related/book-asv/
A Mestre blog concerning their scoring procedure
8. NMR references tables
8.1 Shigemi & Wilmad matched insert color code
| Solvent | Code | Glass Color |
|---|---|---|
| Chloroform | CMS | clear |
| D2O | BMS | clear |
| DMSO | DMS | green |
| Methanol | MMS | blue |
8.2 Reference compounds for chemical shift
| Nuclei | Substance |
Chemical Shift |
|---|---|---|
| 1H | TMS or DSS (aquous solution) | 0 ppm (definition) |
| 13C | TMS | 0 ppm (definition) |
| 19F |
CFCl3 |
0 ppm (definition) |
| 31P | H3PO4 (CH3O)3PO |
0 ppm (definition) 0 ppm |
- a more complete list is available at this page : http://www.nmrnotes.org/NMRPages/refcomps.html
- a good list of 19F reference compounds : http://chemnmr.colorado.edu/manuals/19F_NMR_Reference_Standards.pdf
- an interesting article about standardization of chemical shift in NMR.
9. Useful NMR tricks
9.1 Aromatic solvents to easily increase the spectral resolution
Changing your deuterated solvent from CDCl3 or any other non aromatic solvent by an aromatic deuterated solvent such as Toluene-d8 or Benzene-d6 helps greatly to spread the peaks in the spectrum.
source : http://www.u-of-o-nmr-facility.blogspot.ch/ © Dr. Glenn Facey cited with kind authorization of author.
9.2 some drops of concentrated TFA to simplify spectra
adding some drops of concentrated TFA in NMR samples allows suppressing signals of exchangeable protons inside molecules and generates a broad global peak located halfway between the original peaks and the one of TFA (ca. 15 ppm) - it is therefore generally out of interesting signals.
source : http://www.u-of-o-nmr-facility.blogspot.ch/ © Dr. Glenn Facey cited with kind authorization of author.
10. Kinetic experiments
We have set an automatic kinetic experiment on the nmr1512r machine
Procedure:
1. Acquire a 1D spectrum using the standard refe_1Hzg experiment. This acquisition should be performed on a sample as similar as possible to your kinetic sample
- same compounds and solvent (except a minor component such a catalyser or minor reactant)
- same solvent height
- same tube
2. Check carefully your NS & RGA
3. Once done, type in the TOPSPIN bar command : makekinetic2D.
4. Answer the delays questions (the mentioned delays are the one placed between each loop of acquisition).
5. Type the command "i" to go to the next experiment which is the pseudo 2D kinetic experiment correctly parametrised.
6. Eject your sample and add the missing compound, shake your sample and inject it. Rapidly check manually the shimming (Z1 & Z2) and start the acquisition with "zg"
7. Once the acquisition is finished, you can treat the data with xf2, phase your spectra, correct the baseline and integrate the interesting peaks.
Some tips :
- try to minimise the duration of each acquisition with respect to the global experiment time. It would not be very significant to have each point taking 3 minutes to acquire on a 15 minute experiment. The error on t would be very big. So try to conserve NS as small as possible.
- Since generally kinetic profiles are exponential, it is interesting to acquire more point at the beginning in order to have a good fit. This is possible by defining different delays duration within the experiment.


